A recent study highlights how genetic changes in epigenetic regulator genes shape immune responses in bladder cancer, potentially guiding more personalised therapies.
Understanding why cancer therapies work for some patients but fail for others remains one of modern medicine’s greatest puzzles. As the field of oncology turns increasingly towards personalised medicine, uncovering the molecular and immune signatures that shape treatment response has become crucial. In this context, a new study led by Venugopalareddy Mekala offers fresh insights into how changes in epigenetic regulator genes (epiRGs) influence immune behaviour in bladder cancer.
Published recently in Cancer Medicine, the study maps how specific genetic and epigenetic alterations can alter tumour – immune interactions and even predict which patients are more likely to respond to immunotherapy. By integrating multi-omics data and linking it to immune infiltration and clinical outcomes, Mekala and colleagues provide a valuable framework for refining cancer treatment strategies. Their findings not only deepen our understanding of tumour biology but also hold particular relevance for India, where bladder cancer is an emerging health concern and access to precision oncology tools remains limited.
Bladder cancer continues to pose a major clinician challenge worldwide, with patients often responding differently to treatments. Have you ever wondered why some patients respond well while others don’t?
A team led by Venugopalareddy Mekala, an Indian-origin postdoctoral researcher shed new light on this question. Recently, Mekala and his colleagues at Baylor College of Medicine in the United States published research highlighting how genetic alterations in epigenetic regulator genes (epiRGs) can influence tumour behaviour and the body’s immune response. Epigenetic regulation refers to the control of gene activity and protein production without altering the DNA sequence itself, and epiRGs play a central role in managing these modifications.
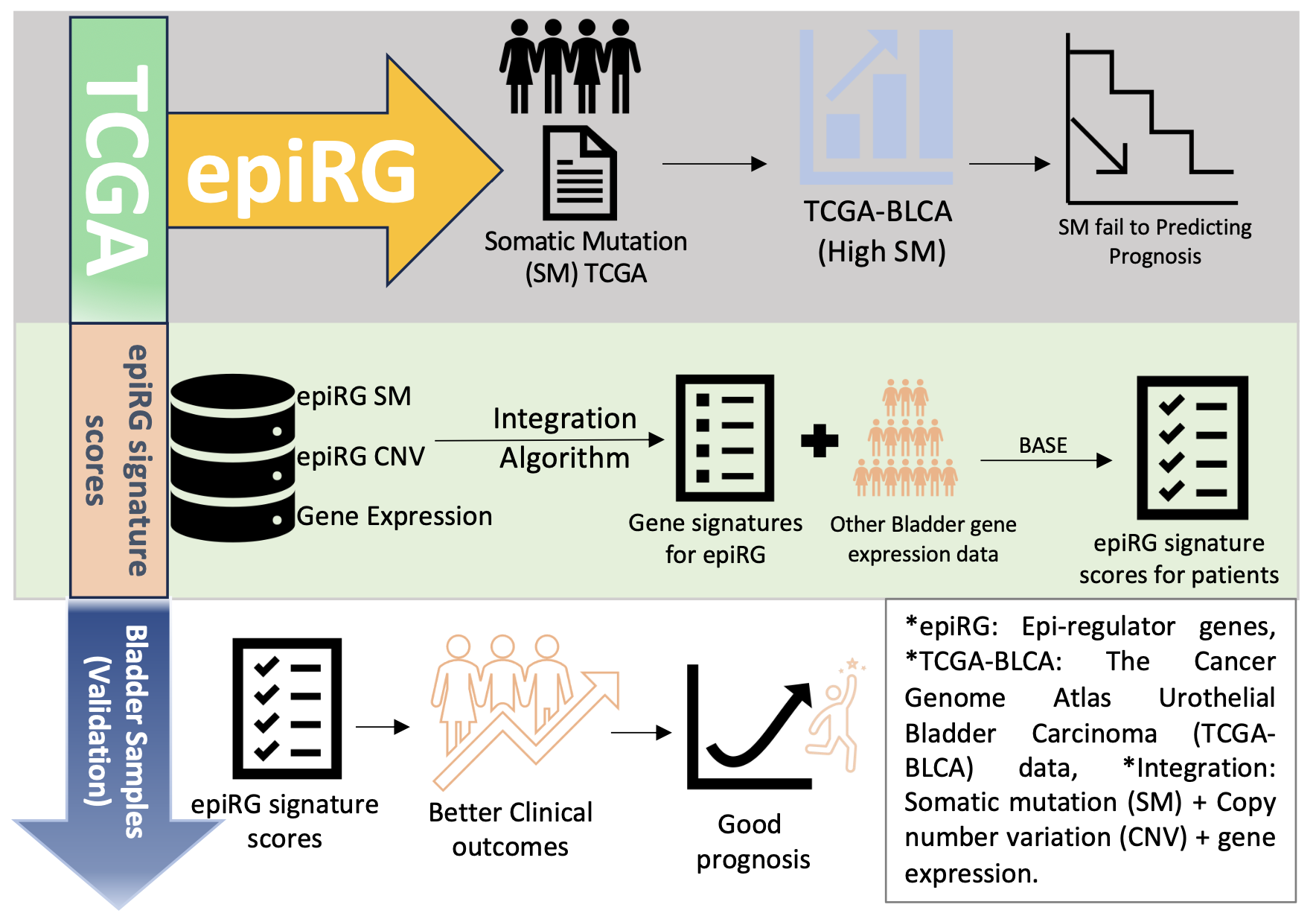
The study, recently published in Cancer Medicine journal, identified 13 signature scores linked to epiRG aberrations in bladder cancer. These scores are generated by using multiple biological integration of bladder patients. These scores go beyond the usual “is this gene mutated?” approach, they capture the functional impact of these changes on the tumour, offering a more nuanced view of how epigenetic disruptions drive cancer progression.
By analysing data from The Cancer Genome Atlas (TCGA) and other datasets, the researchers found that certain mutations, such as CREBBP, EP300, and CHD7, are associated with increased infiltration of immune cells, including CD8+ and CD4+ T cells, macrophages, and dendritic cells. This increased immune activity was often associated with slower tumour growth and better patient prognosis. In contrast, mutations such as PRDM9 amplification showed lower B‑cell infiltration, suggesting possible immune evasion mechanisms by tumours.
The team also explored how these genetic aberrations affect DNA methylation, a key epigenetic mechanism that controls gene expression. Notably, KDM6A mutations were associated with lower global DNA methylation and a unique immune profile, characterised by lower infiltration of most immune cells but slightly higher B‑cell presence. Such findings underscore the complex interplay between genetic changes, epigenetic regulation, and immune activity in bladder cancer.
Using these signature scores, the study assessed patients’ responses to PD-L1 immunotherapy, a widely used immune checkpoint inhibitor. The analysis revealed that patients with high scores in specific epiRGs were more likely to benefit from treatment, suggesting that these scores could serve as biomarkers to predict therapeutic response.
Our findings offer a framework for linking genetic and epigenetic alterations to immune behaviour in bladder tumours”, the authors note.
In the Indian context, these results could contribute to the development of more personalised treatment strategies. Bladder cancer is a serious health issue in India with 18,921 new patients diagnosed and 10,231 bladder cancer-related deaths reported yearly, based on GLOBOCAN 2018. The reported incidence rates are 2.4 and 0.7 per 100,000 for males and females, respectively. It is clear that there is a growing burden, with increasing diversity of risk factors (that include tobacco use, contamination of arsenic and nitrate, and carcinogen exposure at work), which reiterates the necessity of personalised treatment approaches in India.
Although genomics-based approaches are still emerging in India, studies like this highlight the potential of integrating genetic profiling with immunotherapy decisions. Challenges remain, including access to high-throughput sequencing technologies and the need for cost-effective implementation in clinical settings. Nevertheless, the study sets a precedent for similar research and collaborations in India, which could help tailor therapies to patient-specific tumour profiles.
This study highlights the importance of combining genomic and epigenetic insights to better understand tumour biology and enhance patient outcomes. The research not only advances our scientific knowledge of bladder cancer but also opens new avenues for precision medicine globally. By mapping how aberrations in epiRG influence both immune infiltration and DNA methylation patterns, Mekala and colleagues have laid a foundation that could guide future research and clinical strategies. With the growing development of AI-driven tools, the author notes that such technologies could, in time, assist in identifying more personalised treatment options for cancer patients in India and beyond.
indiabioscience.org (Article Sourced Website)
#Mapping #epigenetic #regulator #bladder #cancer #reveals #clues #immunotherapy
