Issued on behalf of Oncolytics Biotech Inc.
VANCOUVER – Baystreet.ca News Commentary – Cancer rates are climbing across the U.S., with stark disparities from state to state. Recent CDC data shows Maine now leads the nation in cancer incidence, while Utah remains the lowest. But survival outcomes aren’t just about geography; they’re increasingly tied to insurance status, with access to immunotherapy varying sharply between private, Medicaid, and uninsured patients. As public funding for cancer research faces deep cuts, potentially slashing the National Cancer Institute’s budget by up to 40%, the burden of innovation is shifting to the private sector. A new wave of biotechs is rising to meet the moment, including Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), Mustang Bio, Inc. (NASDAQ: MBIO), Enliven Therapeutics, Inc. (NASDAQ: ELVN), IN8bio, Inc. (NASDAQ: INAB), and Tango Therapeutics, Inc. (NASDAQ: TNGX).
U.S. cancer death rates may be falling, but the global cancer burden is rising fast. Early-onset diagnoses are increasing at an alarming rate, particularly among younger patients. At the same time, analysts now project the global oncology drug market could surpass US$900 billion in revenue by 2034.
Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC) recently highlighted survival data and an upcoming KOL webinar that together underscore growing expert and clinical support for its virus-based immunotherapy, pelareorep, in hard-to-treat cancers like metastatic pancreatic and breast cancer.
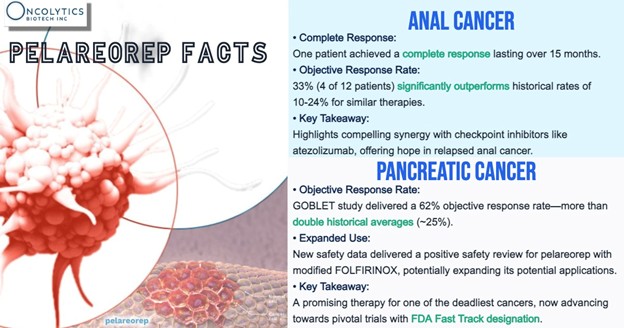
In first-line metastatic pancreatic ductal adenocarcinoma (mPDAC), pelareorep-based treatment regimens have shown a two-year overall survival rate of 21.9% across pooled data from more than 100 patients—more than double the historical benchmark of 9.2%. In a separate single-arm study combining pelareorep with chemotherapy and a checkpoint inhibitor, the objective response rate reached 62% in evaluable patients. No immunotherapy is currently approved in this indication.
“We are no longer in the business of funding proof-of-concept studies,” said Jared Kelly, newly-appointed CEO of Oncolytics. “We have meaningful clinical data in hand—not just signals. The survival benefit across multiple tumor types demands a focused approach to take pelareorep directly into registration-enabling trials. We will use our fast-track status to find the most efficient regulatory path forward this summer to advance our platform in a product technology.”
HR+/HER2- metastatic breast cancer (mBC) has also shown strong survival numbers. In HR+/HER2- mBC, pelareorep extended median overall survival by more than 10 months across two randomized trials. The BRACELET-1 trial also showed median progression-free survival of 12.1 months, nearly double the 6.4 months in the control arm.
The company recently reinforced its leadership bench with two high-profile appointments—naming Jared Kelly as Chief Executive Officer and Andrew Aromando as Chief Business Officer—as it sharpens its focus on late-stage development and strategic transactions. Both Kelly and Aromando played instrumental roles in Ambrx Biopharma’s $2 billion acquisition by Johnson & Johnson, and bring deep experience in value creation, partnerships, and registration pathways in oncology.
“Pelareorep’s clinical data across multiple tumors is striking and represents the potential for a true backbone immunotherapy to address many in-need indications,” said Kelly. “With a renewed focus and sharpened clinical development plan, we believe we will move pelareorep forward effectively and efficiently to a place where potential partners will see the value of a de-risked immunotherapy.”
As CBO, Aromando is now leading global business development and helping shape the company’s corporate, clinical, and regulatory strategies. The leadership tandem is expected to prioritize partnering and expansion opportunities while preserving capital efficiency—a strategy well-suited for pelareorep’s growing clinical profile.
“I’m thrilled to join Oncolytics at such a pivotal moment in its evolution,” said Aromando. “With promising data in difficult-to-treat cancers and a compelling body of clinical evidence in over 1,100 patients, I believe the Company is uniquely positioned to deliver meaningful value to patients and other stakeholders in the near term.”
Oncolytics will host a KOL webinar on July 22 featuring leading GI and immuno-oncology experts to discuss pelareorep’s data and positioning in pancreatic and gastrointestinal cancers. Participating physicians include the GOBLET trial’s primary investigator as well as global leaders in immunotherapy and clinical trial design, underscoring the growing interest in pelareorep’s mechanism and outcomes.
Pelareorep currently holds FDA Fast Track designation in both mPDAC (pancreatic cancer) and HR+/HER2- mBC (breast cancer), with Orphan Drug status for pancreatic cancer in the U.S. and Europe.
Across more than 1,100 patients treated to date, pelareorep has maintained a favorable safety profile, with most adverse events limited to flu-like symptoms. The drug has shown broad synergy with checkpoint inhibitors and chemotherapies and continues to generate immune responses in multiple solid tumor types.
Oncolytics is advancing toward registration-enabling trials and partnership opportunities with a disciplined, investor-aligned approach to growth.
CONTINUED… Read this and more news for Oncolytics Biotech at: https://usanewsgroup.com/2023/10/02/the-most-undervalued-oncolytics-company-on-the-nasdaq/
In other recent industry developments and happenings in the market include:
Mustang Bio, Inc. (NASDAQ: MBIO) has received Orphan Drug Designation from the FDA for its MB-101 CAR T-cell therapy targeting IL13Ra2, a promising approach for treating astrocytomas and glioblastoma. Early clinical results showed that MB-101 was well-tolerated, with 50% of patients achieving stable disease or better, including two complete responses lasting over 7.5 and 66 months.
“We are thrilled that MB-101 received Orphan Drug Designation on time and with a designation that is broader than the indication proposed,” said Manuel Litchman, M.D., President and CEO of Mustang. “The Orphan Drug Designation for MB-101, coupled with the Orphan Drug Designation granted previously for MB-108, is strong validation for our science, as we hope to advance MB-101, in combination with MB-108, as a potential treatment option for patients living with malignant glioma, including patients with recurrent glioblastoma (“GBM”) and high-grade astrocytomas.”
The company is exploring a novel combination with its MB-108 oncolytic virus to convert “cold” tumors into “hot” and improve therapeutic outcomes.
Enliven Therapeutics, Inc. (NASDAQ: ELVN) reported updated data from its Phase 1 ENABLE trial of ELVN-001 in chronic myeloid leukemia (CML), showing a cumulative major molecular response (MMR) rate of 47% by 24 weeks in heavily pretreated patients.
“We are highly encouraged by the ELVN-001 data, specifically as it relates to the consistency of the cumulative and achieved MMR rates as the Phase 1 trial progresses, with more evaluable patients and longer duration of treatment,” said Helen Collins, M.D., Chief Medical Officer of Enliven. “While MMR is the efficacy endpoint in CML, safety and tolerability are equally critical given the chronic nature of the disease. We believe ELVN-001 has the potential to offer best-in-class efficacy and tolerability, which are key attributes for people living with CML. We look forward to sharing additional data in the future.”
The small-molecule kinase inhibitor designed to specifically target BCR::ABL1 gene fusion was well tolerated, with low rates of dose reductions and discontinuations, and no treatment-related arterial occlusive events reported. The company is planning to initiate a pivotal head-to-head Phase 3 trial in 2026.
IN8bio, Inc. (NASDAQ: INAB) reported that a glioblastoma patient treated with its experimental INB-200 therapy has now reached four years of remission, which is a rare outcome in this aggressive cancer type. The patient remains progression-free and has returned to work, highlighting the potential of IN8bio’s gamma-delta T cell approach. Data from the Phase 1 trial show median progression-free survival of 16.1 months, more than double the typical 6.9 months seen with the standard Stupp protocol.
“We are thrilled that our study participant has reached this incredible milestone,” said William Ho, CEO and co-founder, IN8bio. “This type of long-term survival and life changing clinical impact is exactly what we strive to achieve at IN8bio. The current standard-of-care for newly diagnosed GBM has not advanced beyond an overall survival of 14-16 months in over two decades. This is a powerful testament to what’s possible when we harness the unique biology of gamma-delta T cells.”
INB-200 has also demonstrated a strong safety profile and may offer a new avenue of treatment for chemo-resistant brain tumors.
Tango Therapeutics, Inc. (NASDAQ: TNGX) has initiated dosing in a Phase 1/2 trial of TNG462 in combination with RAS inhibitors daraxonrasib or zoldonrasib for patients with RAS-mutant, MTAP-deleted metastatic pancreatic or lung cancers. This population represents a significant unmet need, and preclinical data show compelling synergy between the compounds.
“Almost all MTAP-del pancreatic and approximately 30% of lung cancers have a co-occurring RAS mutation, and preclinical data show strong combination activity of TNG462 with either daraxonrasib or zoldonrasib,” said Adam Crystal, M.D., Ph.D., President, Research and Development of Tango Therapeutics. “Single agent clinical data to date have demonstrated these molecules to be well-tolerated and active in pancreatic cancer and lung cancer, supporting the potential for these combinations to become transformative therapies. The potential of these combinations further strengthens our conviction that TNG462 may play a major role in changing the treatment paradigm for patients with MTAP-deleted cancers.”
TNG462, a potentially best-in-class PRMT5 inhibitor, is also being studied as monotherapy, with new data expected in the second half of 2025. That update is expected to guide a registrational trial in pancreatic cancer and inform future plans in lung cancer.
Source: https://usanewsgroup.com/2024/09/21/is-oncolytics-biotech-the-markets-most-undervalued-cancer-opportunity/
CONTACT:
Baystreet.ca
[email protected]
(250) 999-4849
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. Baystreet.ca is a wholly-owned subsidiary of Baystreet.ca Media Corp. (“BAY”) BAY has been not been paid a fee for Oncolytics Biotech Inc. advertising and/or digital media, but the owner(s) of BAY also own Market IQ Media Group, Inc., which has been paid a fee from the company directly. There may be 3rd parties who may have shares Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of BAY own shares of Oncolytics Biotech Inc. which were purchased in the open market. BAY and all of it’s respective employees, owners and affiliates reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time thereafter without any further notice. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material disseminated by BAY has been approved by the above mentioned company; this is a paid advertisement, and we own shares of the mentioned company that we will sell, and we also reserve the right to buy shares of the company in the open market, or through other investment vehicles. While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.
www.baystreet.ca (Article Sourced Website)
#NextGen #Cancer #Treatments #Gain #Steam #Oncology #Industry #Shakeups
